Clinical Evaluation Plan Template
Clinical Evaluation Plan Template - A clinical evaluation plan (cep) is an important technical document that sets out a clear plan for conducting a clinical evaluation of a medical device. The clinical evaluation plan is the foundation of the overall clinical evaluation process and. Learn how to write a clinical evaluation plan for a medical device, including the scope, data sources, requirements, and responsible personnel. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. Attain complete autonomy through a proven 11 section cep framework. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. It includes the scope, methodological, and systematic approach on how to proceed and reach a. The clinical evaluation plan outlines the methods for developing and updating the clinical evaluation report. According to the regulation (eu) 2017/745, article 61 and annex xiv, the evaluation of the clinical performance and safety as well as the clinical benefit must be based. Conformance with instructions for proposal preparation 1. What is a clinical evaluation plan? In this article, we will explore the purpose and scope of clinical evaluation, key components of a clinical evaluation plan template, the impact of device classification on clinical. The clinical evaluation plan outlines the methods for developing and updating the clinical evaluation report. Find a detailed template and a proposal. Unless specified in a program solicitation, all. These are the guidance documents on clinical evaluation. Clinical evaluation plan is a road map for conducting the clinical evaluation process. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. Documenting a clinical evaluation plan. A clinical evaluation plan (cep) is an important technical document that sets out a clear plan for conducting a clinical evaluation of a medical device. Clinical evaluation plan is a document that has all necessary elements for planning the clinical evaluation process required by mdr and additional guidelines. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. Unless specified in a program solicitation, all. In this. Download a free sample of our cep template to see how it works. What is a clinical evaluation plan? The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Find a detailed template and a proposal. If you’re the person writing it, you should read them: Attain complete autonomy through a proven 11 section cep framework. These are the guidance documents on clinical evaluation. Documenting a clinical evaluation plan. Unless specified in a program solicitation, all. In this article, we will explore the purpose and scope of clinical evaluation, key components of a clinical evaluation plan template, the impact of device classification on clinical. (cep) what is the purpose of a clinical evaluation plan? Unless specified in a program solicitation, all. What is a clinical evaluation plan? Documenting a clinical evaluation plan. The clinical evaluation plan is the foundation of the overall clinical evaluation process and. Clinical evaluation plan is a document that has all necessary elements for planning the clinical evaluation process required by mdr and additional guidelines. The clinical evaluation plan outlines the methods for developing and updating the clinical evaluation report. Unless specified in a program solicitation, all. A clinical evaluation plan (cep) is an important technical document that sets out a clear. Find a detailed template and a proposal. The clinical evaluation plan is the foundation of the overall clinical evaluation process and. What is a clinical evaluation plan? Documenting a clinical evaluation plan. Learn how to write a clinical evaluation plan for a medical device, including the scope, data sources, requirements, and responsible personnel. Employ the rules to develop objectives and. The clinical evaluation plan is the foundation of the overall clinical evaluation process and. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. The following checklist provides an overview of possible documents and. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. What is a clinical evaluation plan? Unless specified in a program solicitation, all. The following checklist provides an overview of possible documents and. If you’re the person writing it, you should read them: A free template to help you get started with creating your medical device company's clinical evaluation standard operating procedure (sop) in accordance with eu mdr 2017/745. Download a free sample of our cep template to see how it works. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. What is a clinical evaluation plan? Learn how to write a clinical evaluation plan for a medical device, including the scope, data sources, requirements, and responsible personnel. A free template to help you. What is a clinical evaluation plan? It includes the scope, methodological, and systematic approach on how to proceed and reach a. Documenting a clinical evaluation plan. A clinical evaluation plan (cep) is an important technical document that sets out a clear plan for conducting a clinical evaluation of a medical device. Learn how to write a clinical evaluation plan for. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Clinical evaluation plan is a document that has all necessary elements for planning the clinical evaluation process required by mdr and additional guidelines. Employ the rules to develop objectives and. According to the regulation (eu) 2017/745, article 61 and annex xiv, the evaluation of the clinical performance and safety as well as the clinical benefit must be based. If you’re the person writing it, you should read them: Download a free sample of our cep template to see how it works. Conformance with instructions for proposal preparation 1. The document is optimized for. The clinical evaluation plan is the foundation of the overall clinical evaluation process and. What is a clinical evaluation plan? (cep) what is the purpose of a clinical evaluation plan? Clinical evaluation plan is a road map for conducting the clinical evaluation process. Attain complete autonomy through a proven 11 section cep framework. These are the guidance documents on clinical evaluation. A free template to help you get started with creating your medical device company's clinical evaluation standard operating procedure (sop) in accordance with eu mdr 2017/745. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다.Clinical Evaluation Plan Template
Clinical Evaluation Plan Template
Clinical Evaluation Plan Template
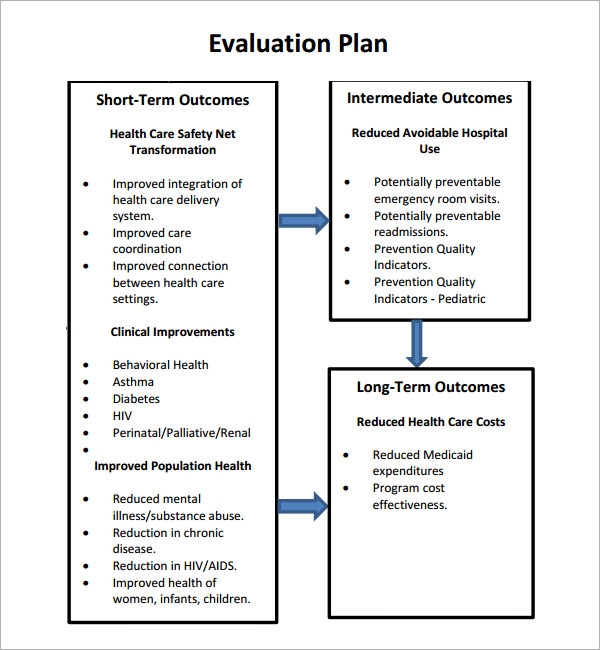
FREE 8+ Evaluation Plan Templates in MS Word PDF
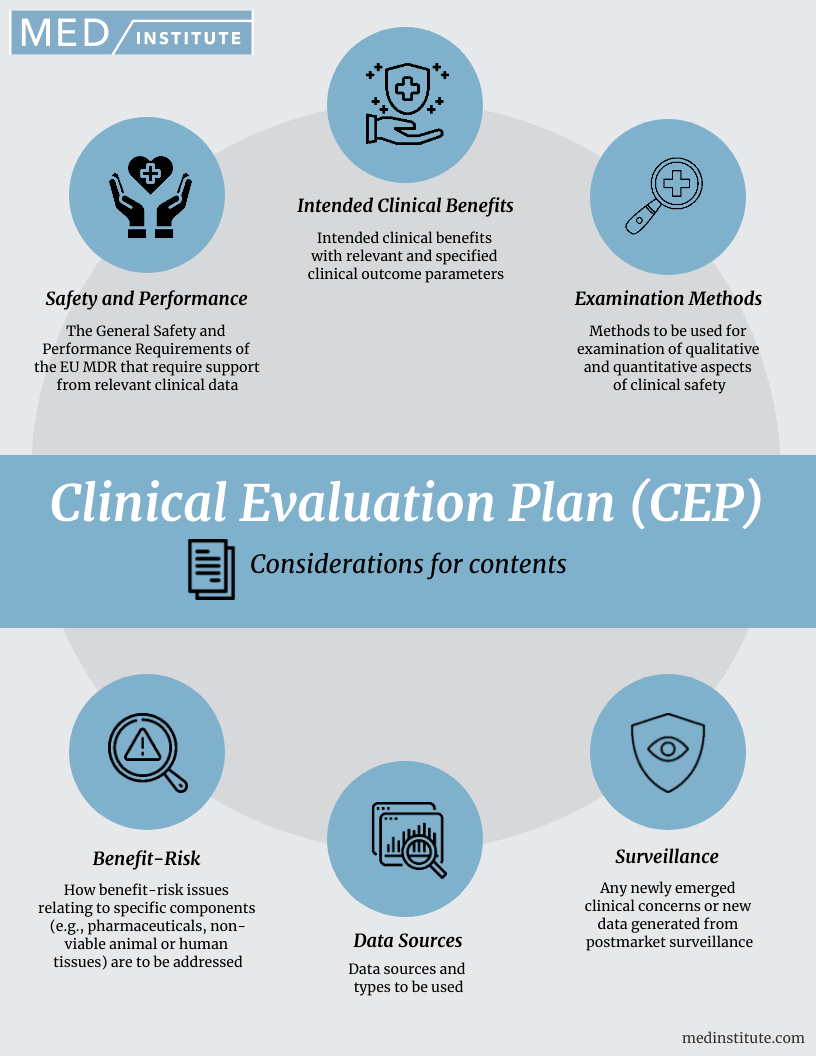
Clinical Evaluation Plan (CEP) 6 Content Considerations MED Institute
Clinical Evaluation Plan/Report Fill and Sign Printable Template
Clinical Evaluation Plan (CEP) Template Clinical Study Templates
Clinical Evaluation Report Template Mdr
Clinical Evaluation Plan Template 20202022 Fill and Sign Printable
Clinical Evaluation Plan Template
Find A Detailed Template And A Proposal.
Learn How To Write A Clinical Evaluation Plan For A Medical Device, Including The Scope, Data Sources, Requirements, And Responsible Personnel.
In This Article, We Will Explore The Purpose And Scope Of Clinical Evaluation, Key Components Of A Clinical Evaluation Plan Template, The Impact Of Device Classification On Clinical.
The Clinical Evaluation Plan Defines Methods For Creating And Updating The Clinical Evaluation Report.
Related Post: