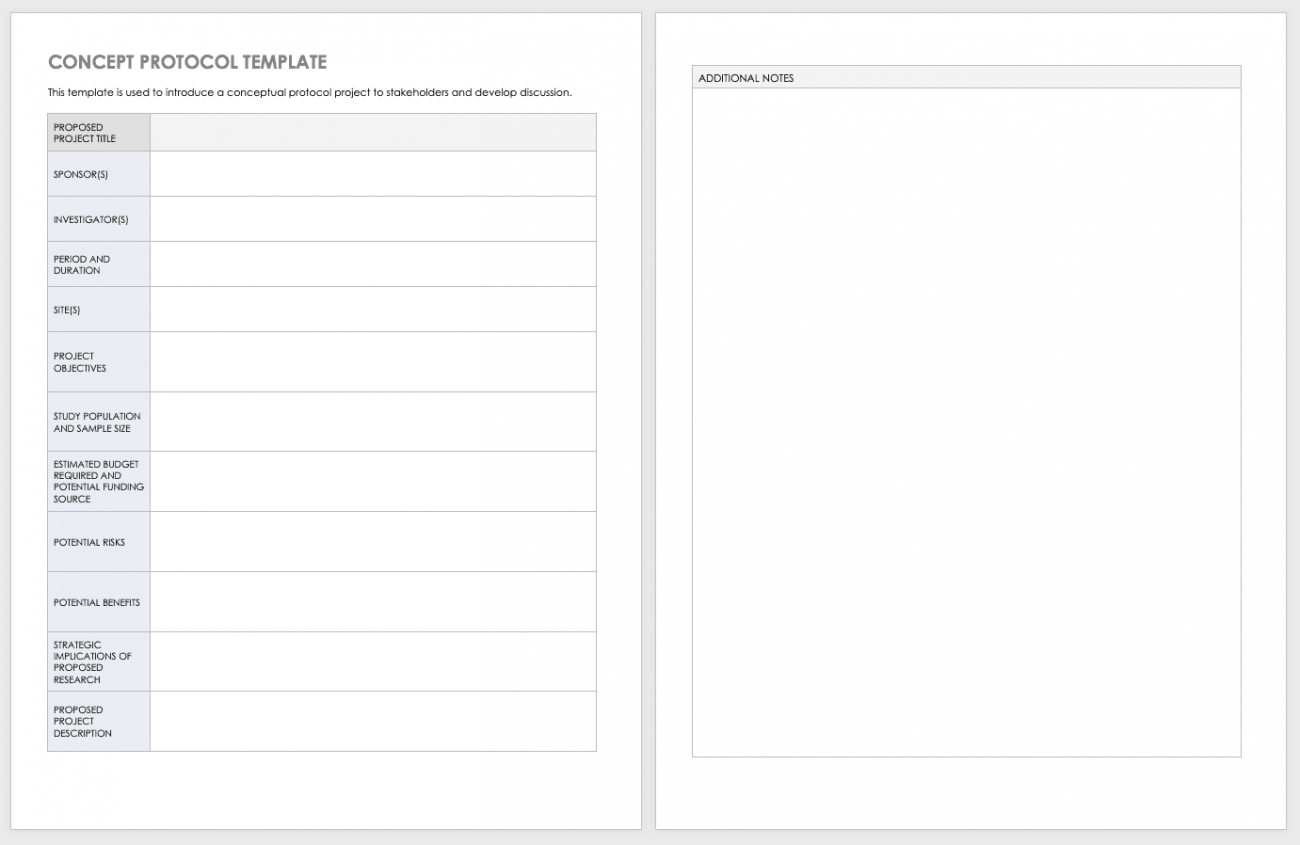
Clinical Study Protocol Template
Clinical Study Protocol Template - Developing a comprehensive clinical trial protocol. The goal of this template is to. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the. Since the protocol and the clinical trial/study report are closely related, further relevant information can be found in the ich guideline for structure and content of clinical study. Describe and provide the results of animal studies, laboratory studies and pilot studies done in the usa or elsewhere, and clinical studies conducted abroad. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Trials is experimenting with a new way of structuring study protocols for randomised trials. Clinical trial protocol eudract number:. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can be associated with different clinical responses or. Phase 2 or 3 clinical trials that require. The protocol is the backbone of your clinical trial, detailing every step of the study. Summarize the known and potential. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. There are three templates to be used for observational research: Multicenter study to evaluate the efficacy and safety of secukinumab in controlling spinal pain in patients with axial spondyloarthritis document type: The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. Clinical trial protocol cena713din01 / nct02989402 a prospective, 16 week, phase iv study to evaluate safety, tolerability and effectiveness in patients with severe dementia of The simple innovation is to include all 51 spirit headings and item identifiers within the protocol. Welcome to global health trials' tools and templates library. The natural history/observational protocol template, the repository protocol template, and the secondary. Trials is experimenting with a new way of structuring study protocols for randomised trials. There are three templates to be used for observational research: The protocol is the backbone of your clinical trial, detailing every step of the study. Summarize the known and potential. Please note that this page has been updated for 2015 following a quality check and review. The goal of this template is to. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. Clinical trial protocol eudract number:. Welcome to global health trials' tools and templates library. After reading, you will understand how to find a relevant clinical. Developing a comprehensive clinical trial protocol. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. Since the protocol and the clinical trial/study report are closely related, further relevant information can be found in the ich guideline for structure and content of clinical study. Many recent studies have shown. Trials is experimenting with a new way of structuring study protocols for randomised trials. The natural history/observational protocol template, the repository protocol template, and the secondary. Section headings and template text formatted in regular type should be included in your protocol document as provided in the template. The proposed guidance document includes a standardized template for manufacturers or other sponsors. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. The natural history/observational protocol template, the repository protocol template, and the secondary. In this blog, you have access to the. The simple innovation is to include all 51 spirit headings and item identifiers within the protocol. Describe and provide the results of animal studies, laboratory studies and pilot studies done in the usa or elsewhere, and clinical studies conducted abroad. Summarize the known and potential. Clinical trial protocol cena713din01 / nct02989402 a prospective, 16 week, phase iv study to evaluate. Developing a comprehensive clinical trial protocol. The goal of this template is to. It ensures consistency across clinical trial. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010 and spirit (standard. Phase 2 or 3 clinical trials that require. Clinical trials are intended in their broadest sense and means any study design that involves an intervention regardless of the nature of that intervention (drug, biologic, device. It ensures consistency across clinical trial. Describe and provide the results of animal studies, laboratory studies and pilot studies done in the usa or elsewhere,. Developing a comprehensive clinical trial protocol. Summarize the known and potential. Multicenter study to evaluate the efficacy and safety of secukinumab in controlling spinal pain in patients with axial spondyloarthritis document type: In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. Phase 2 or 3 clinical trials that require. Describe and provide the results of animal studies, laboratory studies and pilot studies done in the usa or elsewhere, and clinical studies conducted abroad. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. Developing a comprehensive clinical trial protocol. The natural history/observational protocol template, the repository protocol template, and the. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. The simple innovation is to include all 51 spirit headings and item identifiers within the protocol. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the. Clinical trial protocol eudract number:. Trials is experimenting with a new way of structuring study protocols for randomised trials. Phase 2 or 3 clinical trials that require. There are three templates to be used for observational research: Since the protocol and the clinical trial/study report are closely related, further relevant information can be found in the ich guideline for structure and content of clinical study. Describe and provide the results of animal studies, laboratory studies and pilot studies done in the usa or elsewhere, and clinical studies conducted abroad. Multicenter study to evaluate the efficacy and safety of secukinumab in controlling spinal pain in patients with axial spondyloarthritis document type: Summarize the known and potential. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. Section headings and template text formatted in regular type should be included in your protocol document as provided in the template. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Developing a comprehensive clinical trial protocol. Clinical trial protocol cena713din01 / nct02989402 a prospective, 16 week, phase iv study to evaluate safety, tolerability and effectiveness in patients with severe dementia ofClinical Study Protocol Template
Clinical Study Protocol Template
Free Clinical Trial Templates Smartsheet
Clinical Study Protocol (CSP) Template Clinical Study Templates
instructions for clinical research protocol template Doc Template
CLINICAL TRIAL PROTOCOL TEMPLATE DEVELOPING CLINICAL TRIAL
Clinical Trial Protocol Template Word
Clinical Study Protocol Template
Clinical Trial Protocol Synopsis Template
Clinical Study Protocol PowerPoint and Google Slides Template PPT Slides
The Protocol Is The Backbone Of Your Clinical Trial, Detailing Every Step Of The Study.
The Goal Of This Template Is To.
Many Recent Studies Have Shown That Highly Selective Biologic Drugs Are Not Effective In Every Patient And That Variations In The Genome Can Be Associated With Different Clinical Responses Or.
Welcome To Global Health Trials' Tools And Templates Library.
Related Post: