Clinical Study Report Template
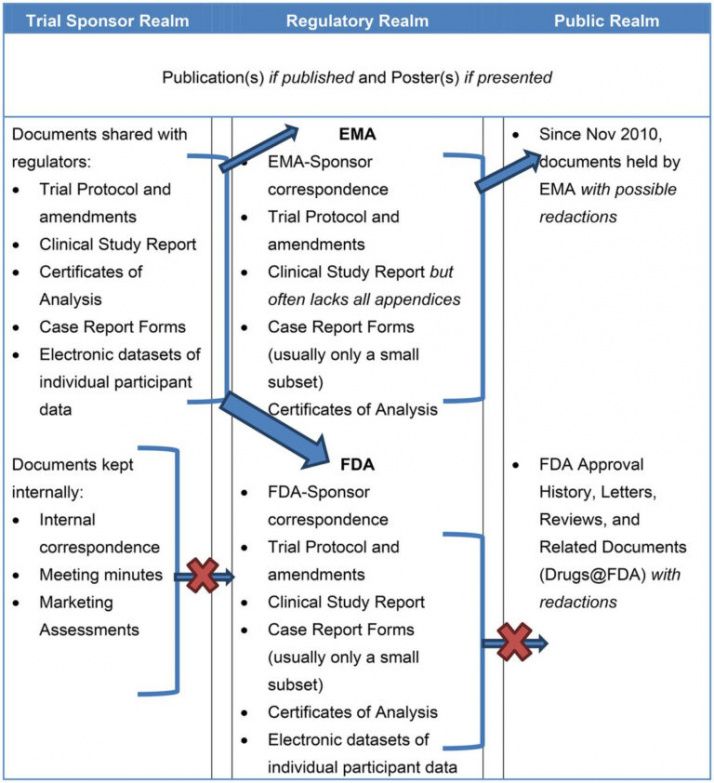
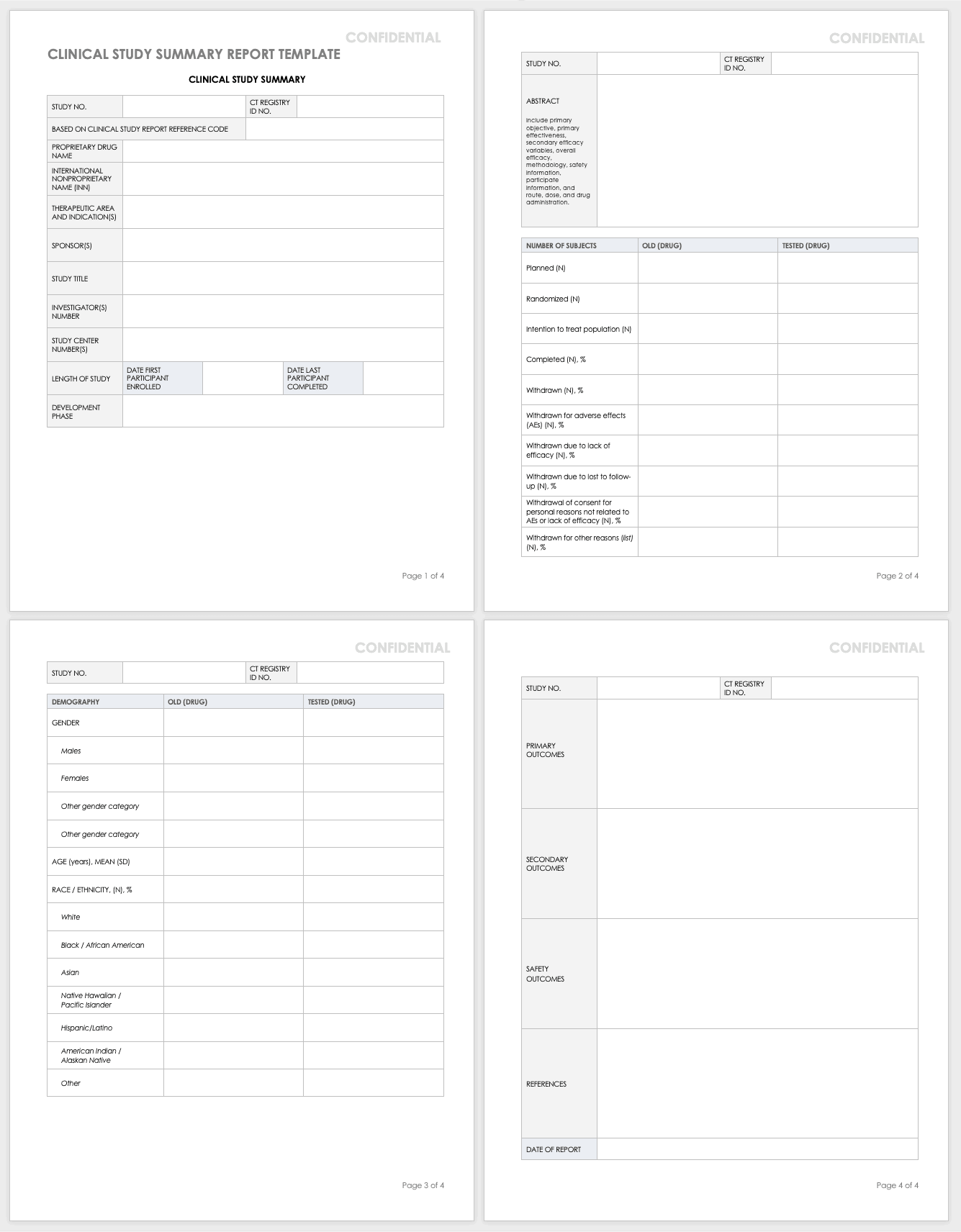
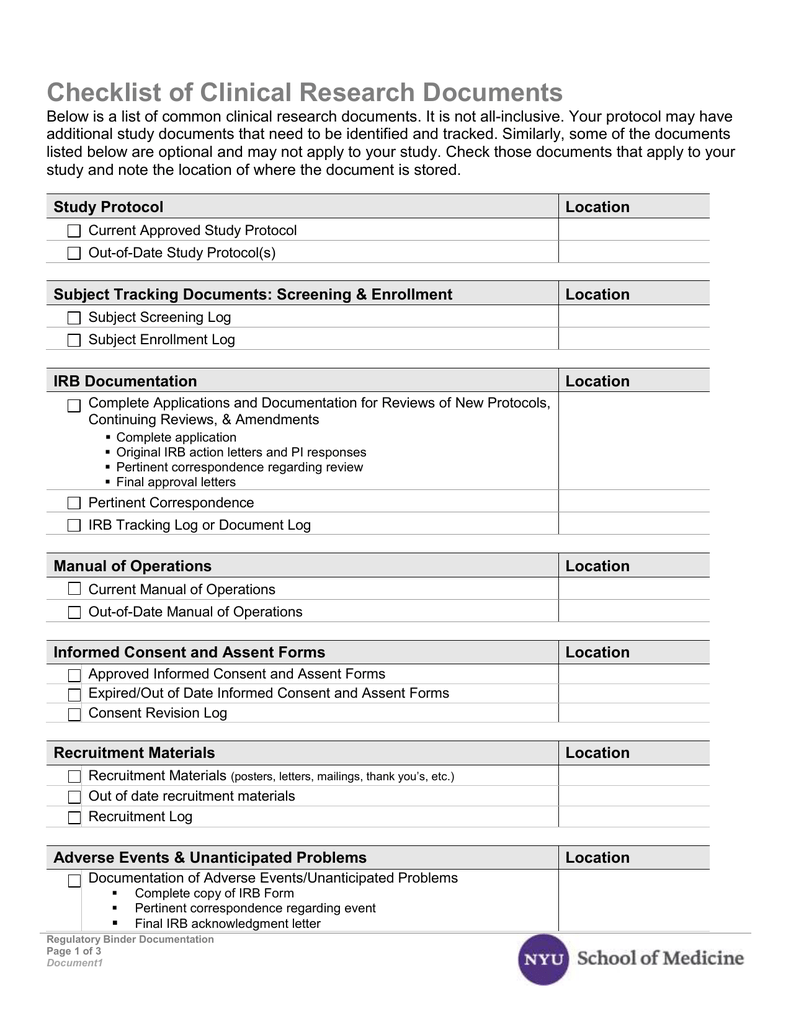
Clinical Study Report Template - It covers the general format, the elements of the report, and the. The schedule of assessments (refer to the study protocol) gives an overview of the trial procedures. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as an adjunctive therapy for normal tension. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. The common clinical study report (csr) template provides a common, streamlined structure to report data, adhere to global requirements, enable disclosures, and seamlessly integrate with. Find protocol, data management, and monitoring templates for clinical trials funded by niams. Signature pages for clinical study report i have read this report and confirm that to the best of my knowledge it accurately describes the conduct and results of the study. Example report card with individualized feedback and guidance on the transparency of a trial. Developing a comprehensive clinical trial protocol. The clinical trial management system (ctms) market, which is closely related to cdms, is projected to grow at a cagr of 11.4% through 2030, driven by the rising adoption of digital. Read together with international conference on. The common clinical study report (csr) template provides a common, streamlined structure to report data, adhere to global requirements, enable disclosures, and seamlessly integrate with. It covers topics such as title page, synopsis, ethics, study objectives,. Patients should attend all visits on the designated day or as close to it as possible. It covers topics such as. Developing a comprehensive clinical trial protocol. The clinical documentation improvement (cdi) market is projected to grow from usd 4,205 million in 2024 to usd 7,499.52 million by 2032, with a compound annual growth. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of pharmaceuticals for human use. Safety and efficacy of brimonidine posterior segment drug delivery system in patients with geographic atrophy secondary to age‐related macular degeneration It covers topics such as title page, synopsis, ethics, study objectives,. It covers the general format, the elements of the report, and the. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of pharmaceuticals for human use. Original research reports, preferably clinical trials or systematic reviews that address virtually any aspect. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. It covers topics such as. Signature pages for clinical study report i have read this report and confirm that to the best of my knowledge it accurately describes the conduct and results of the study. This is. The schedule of assessments (refer to the study protocol) gives an overview of the trial procedures. Example report card with individualized feedback and guidance on the transparency of a trial. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and more. Download free clinical trial templates for your clinical. Download free clinical trial templates for your clinical research, available in sharepoint, word, excel, and microsoft project formats. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. The template is compatible with the clinical study protocol template and can be used for any. This document provides a note for guidance on the. Download free clinical trial templates for your clinical research, available in sharepoint, word, excel, and microsoft project formats. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. The clinical documentation improvement (cdi) market is projected to grow from usd 4,205 million in 2024 to usd 7,499.52 million by 2032, with a compound. The sections of this cer template include. The schedule of assessments (refer to the study protocol) gives an overview of the trial procedures. It covers the general format, the elements of the report, and the. It covers topics such as. The clinical documentation improvement (cdi) market is projected to grow from usd 4,205 million in 2024 to usd 7,499.52 million. Download the clinical study report template. The template is compatible with the clinical study protocol template and can be used for any. This statistical analysis plan (sap) details comprehensive, technical specifications of the statistical analyses of the efficacy and safety data outlined and/or specified in the final protocol. Read together with international conference on. The schedule of assessments (refer to. Example report card with individualized feedback and guidance on the transparency of a trial. The template is compatible with the clinical study protocol template and can be used for any. Signature pages for clinical study report i have read this report and confirm that to the best of my knowledge it accurately describes the conduct and results of the study.. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Download a ms word template for a clinical study report (csr) that follows ich e3 guidelines. This clinical evaluation report. The clinical trial management system (ctms) market, which is closely related to cdms, is projected to grow at a cagr of 11.4% through 2030, driven by the rising adoption of digital. Signature pages for clinical study report i have read this report and confirm that to the best of my knowledge it accurately describes the conduct and results of the. Clinical study protocol (csp) clinical study report (csr). This document provides recommendations for the structure and content of clinical study reports submitted to the fda. Example report card with individualized feedback and guidance on the transparency of a trial. The common clinical study report (csr) template provides a common, streamlined structure to report data, adhere to global requirements, enable disclosures, and seamlessly integrate with. The schedule of assessments (refer to the study protocol) gives an overview of the trial procedures. This clinical evaluation report (cer) template is specifically designed to be used in conjunction with the clinical evaluation plan (cep) template. This statistical analysis plan (sap) details comprehensive, technical specifications of the statistical analyses of the efficacy and safety data outlined and/or specified in the final protocol. It covers topics such as. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and more. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. Read together with international conference on. Download the clinical study report template. It covers the general format, the elements of the report, and the. The sections of this cer template include. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. The clinical trial management system (ctms) market, which is closely related to cdms, is projected to grow at a cagr of 11.4% through 2030, driven by the rising adoption of digital.Free Clinical Trial Templates Smartsheet
Clinical Trial Report Template TEMPLATES EXAMPLE TEMPLATES EXAMPLE
Clinical Study Report Template PDF Sample Stableshvf
Best Clinical Study Report Template Excel Example Stableshvf
Clinical Study Report (CSR) Template Clinical Study Templates
Clinical Research Report Synopsis Templates At for Clinical Trial
Free Clinical Trial Templates Smartsheet
Clinical Study Report (CSR), Protocol (CSP), and Synopsis (CSPS
Clinical Study Report Cardiovascular Diseases Coronary Artery Disease
Clinical Evaluation Report Template
This Document Provides A Harmonised Tripartite Guideline For The Structure And Content Of Clinical Study Reports For Registration Of Pharmaceuticals For Human Use.
The Template Is Compatible With The Clinical Study Protocol Template And Can Be Used For Any.
Developing A Comprehensive Clinical Trial Protocol.
The Clinical Documentation Improvement (Cdi) Market Is Projected To Grow From Usd 4,205 Million In 2024 To Usd 7,499.52 Million By 2032, With A Compound Annual Growth.
Related Post: