Company Core Safety Information Template
Company Core Safety Information Template - The company core data sheet (ccds) of an organization is the central document prepared by the applicant (the marketing authorization holder [mah]), which contains all the essential. The company core data sheet (ccds) serves as a key document representing the pharmaceutical company’s position on a generic medicinal product. Every business needs health and. A safety policy statement is a document that declares your company’s commitment to safety. All relevant safety information contained within a company core data sheet or smpc prepared by the marketing authorisation holder (mah). Participants will work with a robust ccds template and detailed user manual that can be later adopted in part or full by their organizations. Here are 10 free health and safety templates you can edit, fill in, download, and use. Company core safety information (ccsi). Products will harmonize their practices regarding core safety information (csi) that their internal, central core data sheets must contain. A practical option for the purpose of periodic reporting is for each mah to use, as a reference, the safety information contained within its central document (ccds), which will be referred to as. The company core data sheet (ccds) serves as a key document representing the pharmaceutical company’s position on a generic medicinal product. As introduced by cioms working group 11 on periodic. A company core data sheet (ccds) serves as the fundamental document in pharmaceuticals, outlining critical data about a drug's properties, usage, safety, and efficacy guidelines. All relevant safety information contained within a company core data sheet or smpc prepared by the marketing authorisation holder (mah). Development of a global company core data sheet (ccds) for harmonisation of regulatory labeling. A safety policy statement is a document that declares your company’s commitment to safety. Every business needs health and. The company core data sheet (ccds) of an organization is the central document prepared by the applicant (the marketing authorization holder [mah]), which contains all the essential. Company core safety information (ccsi) that their internal, central company core data sheets for a marketed drug must contain. Created by health and safety experts, ready for your work. The purpose of having a ccds firstly is to align the labeling of any said drug product across the globe and to have the “reference safety information” for the assessment of the aggregate. A safety plan template is a document, which is designed to be modified according to the particular needs of the organization, that contains the procedures and measures. Created by health and safety experts, ready for your work. As introduced by cioms working group ii, on. Participants will work with a robust ccds template and detailed user manual that can be later adopted in part or full by their organizations. Discloses company’s view of safety profile to regulators. Company core safety information (ccsi) is a term used in. It recognizes safety as a company core value, on a par with production and quality. “all relevant safety information contained in the company core data sheet prepared by the marketing authorisation holder (mah) and which the mah. Products will harmonize their practices regarding core safety information (csi) that their internal, central core data sheets must contain. A practical option for. Company core safety information (ccsi). Here are 10 free health and safety templates you can edit, fill in, download, and use. Discloses company’s view of safety profile to regulators. As introduced by cioms working group ii, on. A safety plan template is a document, which is designed to be modified according to the particular needs of the organization, that contains. As introduced by cioms working group 11 on periodic. Products will harmonize their practices regarding core safety information (csi) that their internal, central core data sheets must contain. A company core data sheet (ccds) serves as the fundamental document in pharmaceuticals, outlining critical data about a drug's properties, usage, safety, and efficacy guidelines. Development of a global company core data. Products will harmonize their practices regarding core safety information (csi) that their internal, central core data sheets must contain. The company core data sheet (ccds) of an organization is the central document prepared by the applicant (the marketing authorization holder [mah]), which contains all the essential. A practical option for the purpose of periodic reporting is for each mah to. A company core data sheet (ccds) serves as the fundamental document in pharmaceuticals, outlining critical data about a drug's properties, usage, safety, and efficacy guidelines. Participants will work with a robust ccds template and detailed user manual that can be later adopted in part or full by their organizations. Products will harmonize their practices regarding core safety information (csi) that. Products will harmonize their practices regarding core safety information (csi) that their internal, central core data sheets must contain. Participants will work with a robust ccds template and detailed user manual that can be later adopted in part or full by their organizations. Development of a global company core data sheet (ccds) for harmonisation of regulatory labeling. The company core. Every business needs health and. The purpose of having a ccds firstly is to align the labeling of any said drug product across the globe and to have the “reference safety information” for the assessment of the aggregate. Lets regulators expect attempts to implement a certain set of safety. Here are 10 free health and safety templates you can edit,. The company core data sheet (ccds) of an organization is the central document prepared by the applicant (the marketing authorization holder [mah]), which contains all the essential. The purpose of having a ccds firstly is to align the labeling of any said drug product across the globe and to have the “reference safety information” for the assessment of the aggregate.. Discloses company’s view of safety profile to regulators. Participants will work with a robust ccds template and detailed user manual that can be later adopted in part or full by their organizations. Created by health and safety experts, ready for your work. Development of a global company core data sheet (ccds) for harmonisation of regulatory labeling. Lets regulators expect attempts to implement a certain set of safety. Here are 10 free health and safety templates you can edit, fill in, download, and use. A company core data sheet (ccds) serves as the fundamental document in pharmaceuticals, outlining critical data about a drug's properties, usage, safety, and efficacy guidelines. “all relevant safety information contained in the company core data sheet prepared by the marketing authorisation holder (mah) and which the mah. A safety policy statement is a document that declares your company’s commitment to safety. The purpose of having a ccds firstly is to align the labeling of any said drug product across the globe and to have the “reference safety information” for the assessment of the aggregate. As introduced by cioms working group 11 on periodic. As introduced by cioms working group ii, on. A practical option for the purpose of periodic reporting is for each mah to use, as a reference, the safety information contained within its central document (ccds), which will be referred to as. Company core safety information (ccsi) is a term used in the field of pharmacovigilance and drug safety to refer to a standardized and comprehensive set of safety. A safety plan template is a document, which is designed to be modified according to the particular needs of the organization, that contains the procedures and measures that the company. Products will harmonize their practices regarding core safety information (csi) that their internal, central core data sheets must contain.FREE 31+ Data Sheet Templates in MS Word PDF
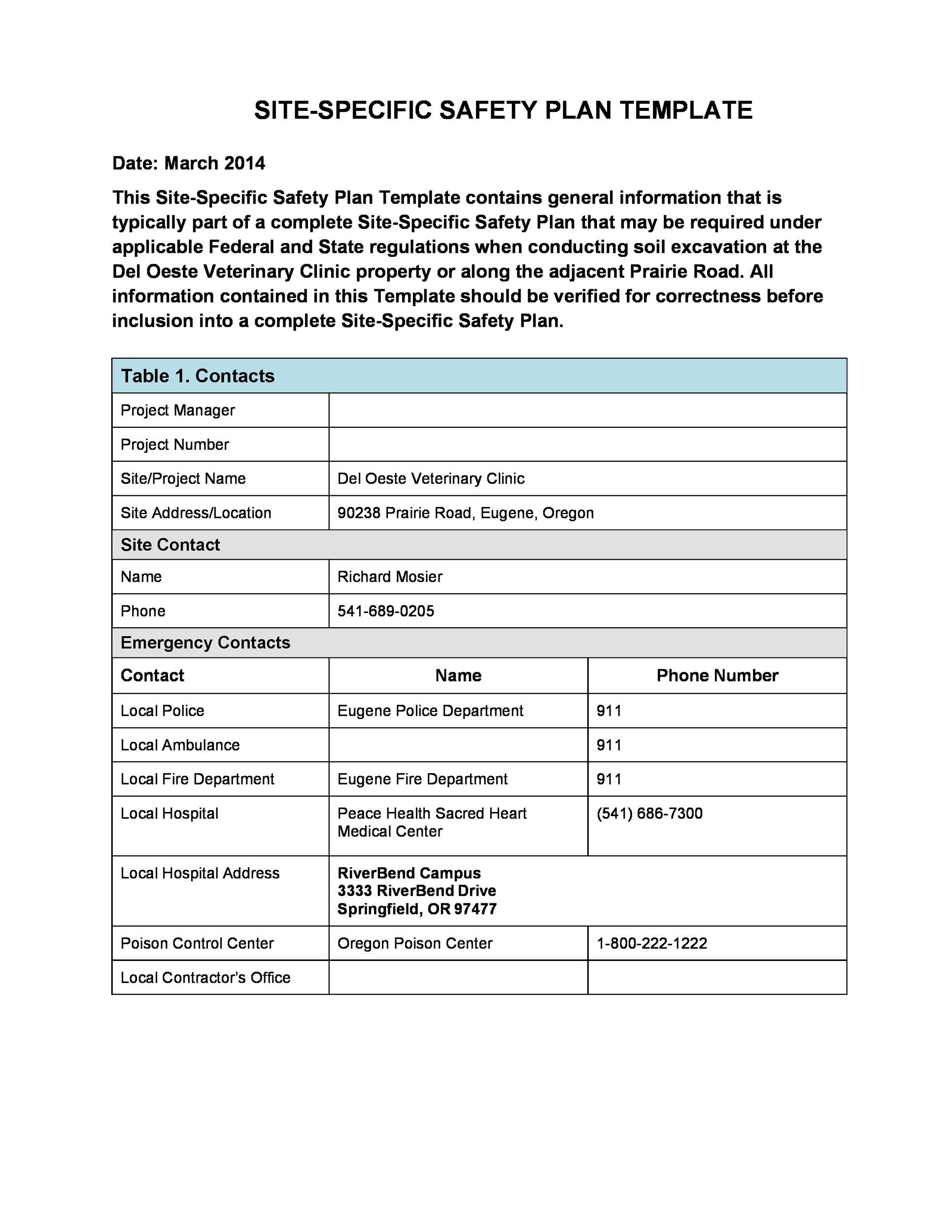
8+ Sample Safety Plan Templates Sample Templates
Health and safety plan Templates 14+ Docs, Free Downloads
CORESafety Infographic Five Safety Hazards to Anticipate and Handle
CCDS and local labeling text. CCDS, Company Core Data Sheet. Download
46 Great Safety Plan Templates (Construction, Site Specific, Patient
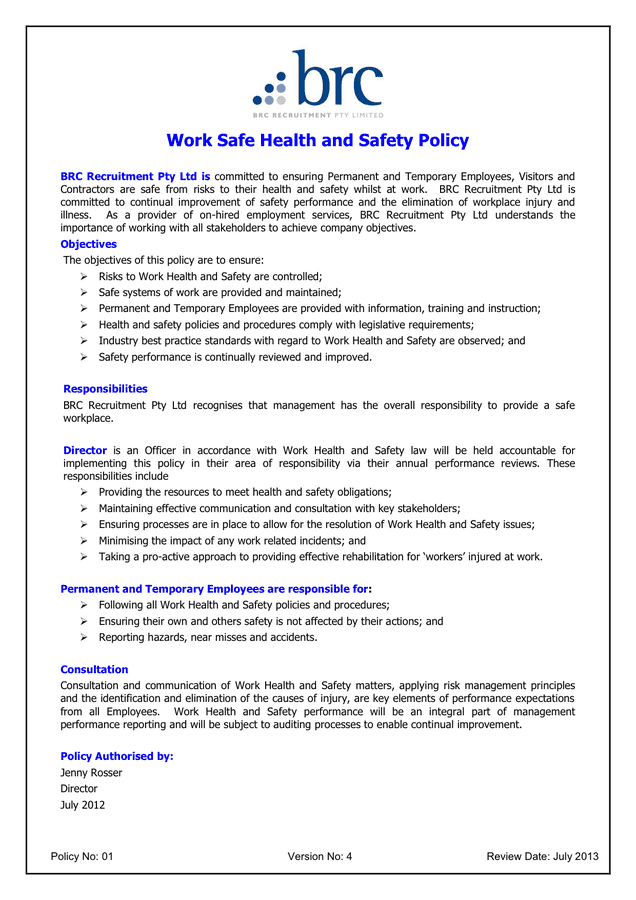
HEALTH AND SAFETY POLICY Template in Word and Pdf formats
Safety Report Templates at
FREE 14+ Safety Manual Samples in PDF MS Word
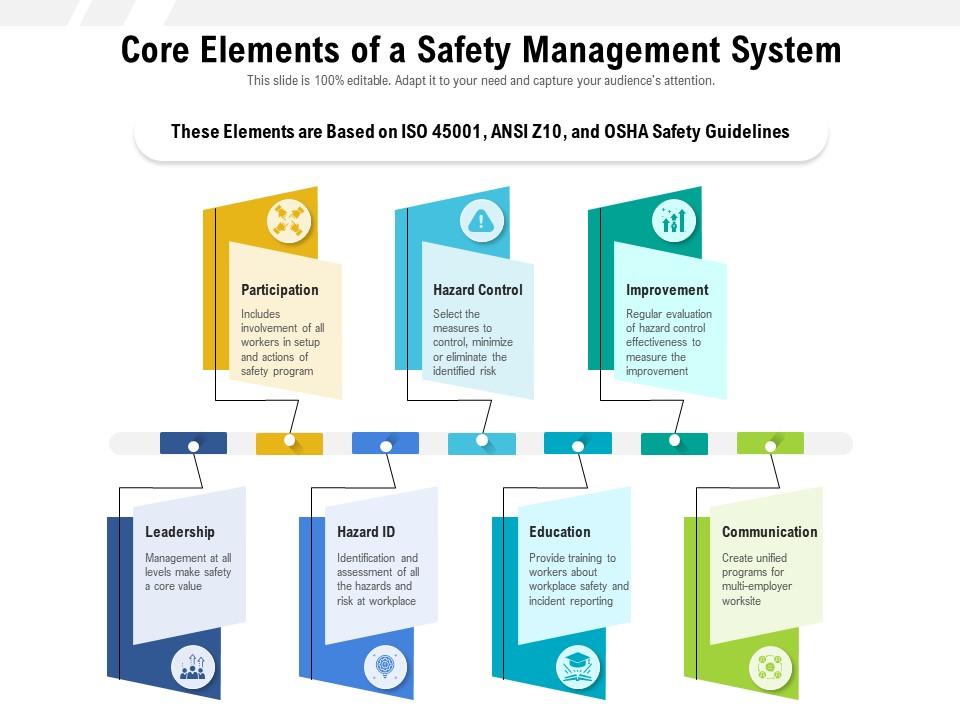
Core Elements Of A Safety Management System Presentation Graphics
The Company Core Data Sheet (Ccds) Serves As A Key Document Representing The Pharmaceutical Company’s Position On A Generic Medicinal Product.
It Recognizes Safety As A Company Core Value, On A Par With Production And Quality.
Company Core Safety Information (Ccsi) That Their Internal, Central Company Core Data Sheets For A Marketed Drug Must Contain.
Every Business Needs Health And.
Related Post: