Iqcp Template
Iqcp Template - Performing a risk assessment will facilitate the. The cornerstone of iqcp is identifying, evaluating, and controlling potential sources of error relevant to the individual laboratory. Presentation on iqcp implementation in microbiology labs, covering risk assessment, qc planning, and cms/clia standards. The individualized quality control plan (iqcp) option offers the laboratory flexibility for meeting regulatory qc requirements. This powerpoint presentation covers the cap checklist requirements,. See a sample iqcp templa… Find resources, examples and tips for evaluating. Download templates, risk assessment guidance and educational materials for clsi exempt. Evaluate the impact of iqcp on quality control. The document provides a template for implementing an individualized quality control plan (iqcp) for commercially prepared microbiological media, emphasizing the need for laboratories to. The iqcp option offers your laboratory flexibility for meeting regulatory qc requirements appropriate for. Identify components that should be included in an iqcp 2. See a sample iqcp templa… Quality control plan (iqcp) for your unique testing environment and your patients. The document provides a template for implementing an individualized quality control plan (iqcp) for commercially prepared microbiological media, emphasizing the need for laboratories to. This powerpoint presentation covers the cap checklist requirements,. The individualized quality control plan (iqcp) option offers the laboratory flexibility for meeting regulatory qc requirements. A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp) templates and created the first such template for. Iqcp stands for individualized quality control plan and is the alternative clia quality control (qc) option that will provide for equivalent quality testing to meet the clia regulations for. Laboratories should modify this template and examples to support their practice. Performing a risk assessment will facilitate the. Find resources, examples and tips for evaluating. This powerpoint presentation covers the cap checklist requirements,. Laboratories should modify this template and examples to support their practice. Analyze the process of creating an iqcp’s step by step, using iqcp examples 3. Laboratories should modify this template and examples to support their practice. Identify components that should be included in an iqcp 2. To facilitate completion of a risk assessment (ra) and adherence with the individualized quality control plan (iqcp) option, abbott point of care provides a guide to aid. Iqcp permits the laboratory to customize its qc plan according to test. Evaluate the impact of iqcp on quality control. Download templates, risk assessment guidance and educational materials for clsi exempt. Iqcp permits the laboratory to customize its qc plan according to test method and use, environment, and personnel competency while providing for equivalent quality testing. Iqcp stands for individualized quality control plan and is the alternative clia quality control (qc) option. Iqcp permits the laboratory to customize its qc plan according to test method and use, environment, and personnel competency while providing for equivalent quality testing. See a sample iqcp templa… Find resources, examples and tips for evaluating. Download templates, risk assessment guidance and educational materials for clsi exempt. The individualized quality control plan (iqcp) option offers the laboratory flexibility for. Quality control plan (iqcp) for your unique testing environment and your patients. Identify components that should be included in an iqcp 2. Learn how to apply individualized quality control plan (iqcp) for microbiology tests that are not performed each day of patient testing. To facilitate completion of a risk assessment (ra) and adherence with the individualized quality control plan (iqcp). Learn about the individualized quality control plan (iqcp) option for laboratories, its eligibility, elements, and benefits. A unique iqcp should be implemented for each separate identification method or system. Analyze the process of creating an iqcp’s step by step, using iqcp examples 3. The individualized quality control plan (iqcp) option offers the laboratory flexibility for meeting regulatory qc requirements. Performing. To facilitate completion of a risk assessment (ra) and adherence with the individualized quality control plan (iqcp) option, abbott point of care provides a guide to aid. Find iqcp resources and documentation for thermo fisher scientific microbiology products. Analyze the process of creating an iqcp’s step by step, using iqcp examples 3. Learn how to develop an iqcp. A distinguished. Analyze the process of creating an iqcp’s step by step, using iqcp examples 3. Find iqcp resources and documentation for thermo fisher scientific microbiology products. A unique iqcp should be implemented for each separate identification method or system. Performing a risk assessment will facilitate the. Iqcp permits the laboratory to customize its qc plan according to test method and use,. The iqcp option offers your laboratory flexibility for meeting regulatory qc requirements appropriate for. Learn about the individualized quality control plan (iqcp) option for laboratories, its eligibility, elements, and benefits. Find iqcp resources and documentation for thermo fisher scientific microbiology products. A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp) templates and created the. Identify components that should be included in an iqcp 2. Performing a risk assessment will facilitate the. A unique iqcp should be implemented for each separate identification method or system. Learn how to develop an iqcp. Learn about the individualized quality control plan (iqcp) option for laboratories, its eligibility, elements, and benefits. Presentation on iqcp implementation in microbiology labs, covering risk assessment, qc planning, and cms/clia standards. The iqcp option offers your laboratory flexibility for meeting regulatory qc requirements appropriate for. Laboratories should modify this template and examples to support their practice. This powerpoint presentation covers the cap checklist requirements,. Find resources, examples and tips for evaluating. Download templates, risk assessment guidance and educational materials for clsi exempt. Analyze the process of creating an iqcp’s step by step, using iqcp examples 3. Iqcp permits the laboratory to customize its qc plan according to test method and use, environment, and personnel competency while providing for equivalent quality testing. Performing a risk assessment will facilitate the. The individualized quality control plan (iqcp) option offers the laboratory flexibility for meeting regulatory qc requirements. The document provides a template for implementing an individualized quality control plan (iqcp) for commercially prepared microbiological media, emphasizing the need for laboratories to. Quality control plan (iqcp) for your unique testing environment and your patients. Identify components that should be included in an iqcp 2. Evaluate the impact of iqcp on quality control. The cornerstone of iqcp is identifying, evaluating, and controlling potential sources of error relevant to the individual laboratory. Find iqcp resources and documentation for thermo fisher scientific microbiology products.IQCP GUIDELINES and TEMPLATE FOR GETTING STARTED Doc Template pdfFiller
Individualized Quality Control Plan (IQCP) Is It Value Doc
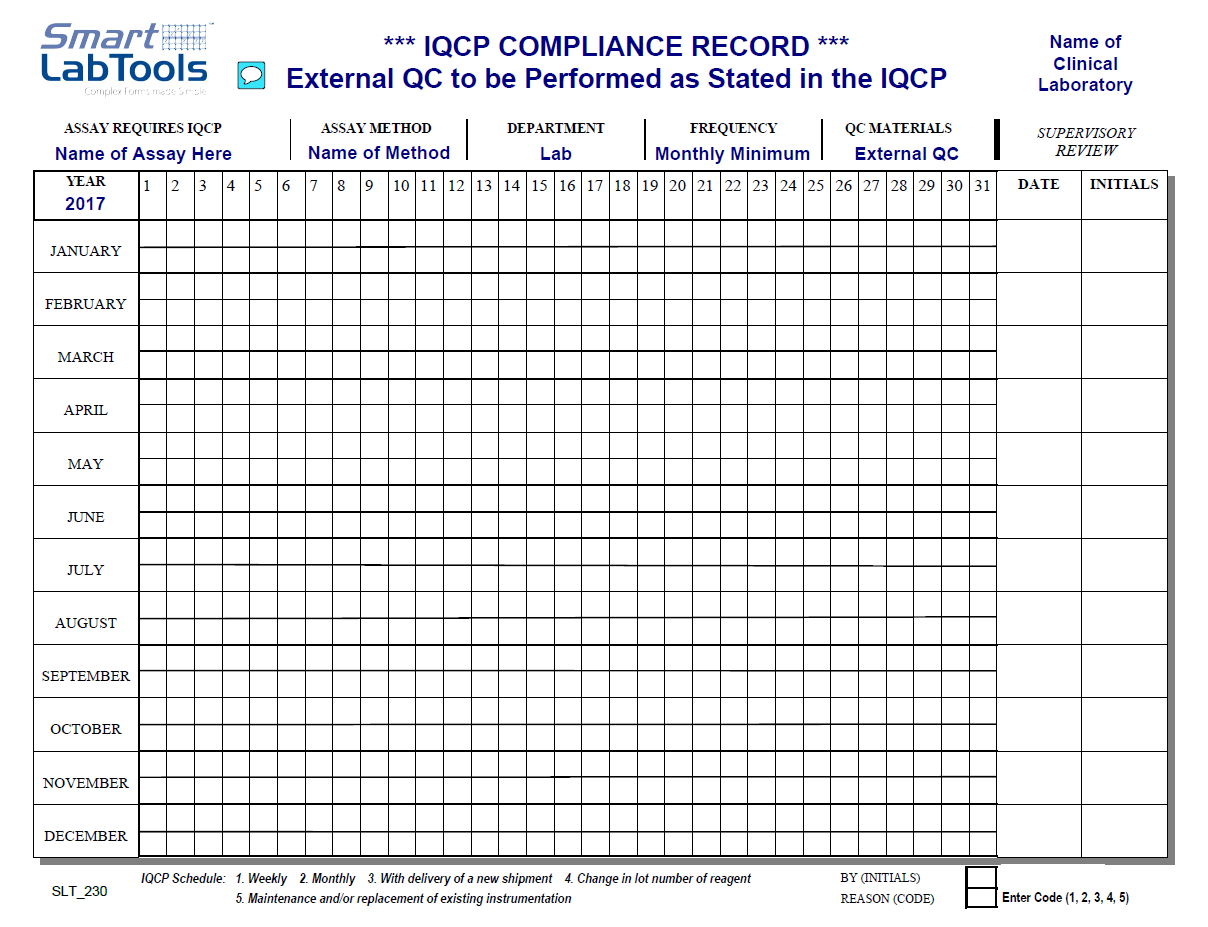
SmartLabTools SLT_IQCP Review Forms
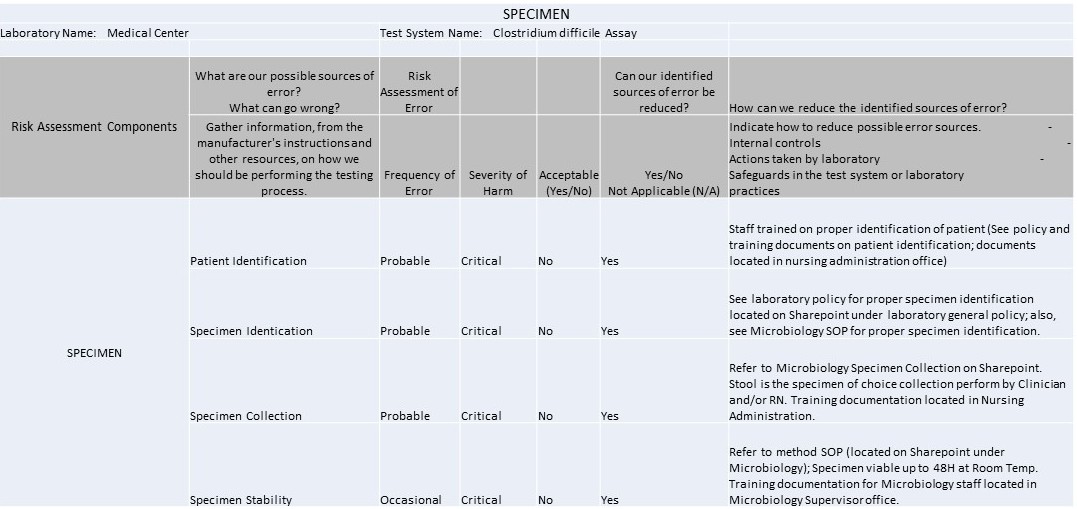
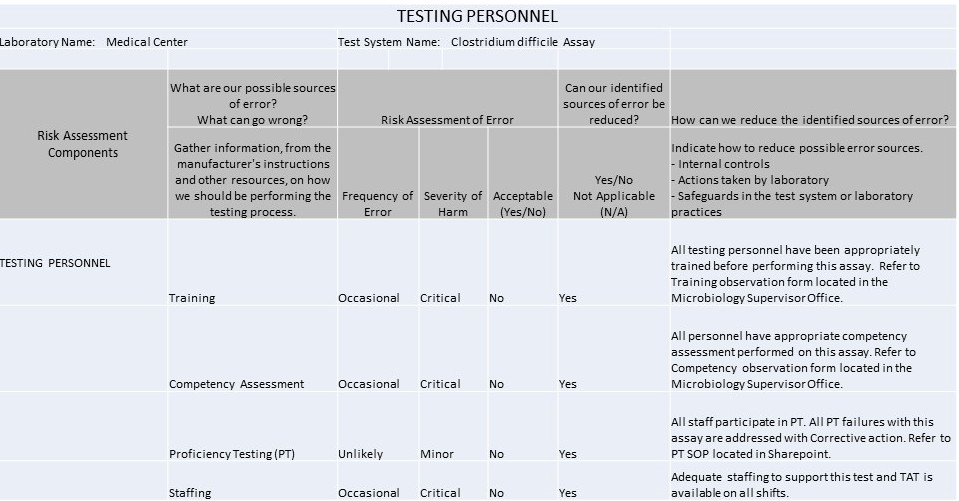
Example IQCP for C. Difficile Westgard QC
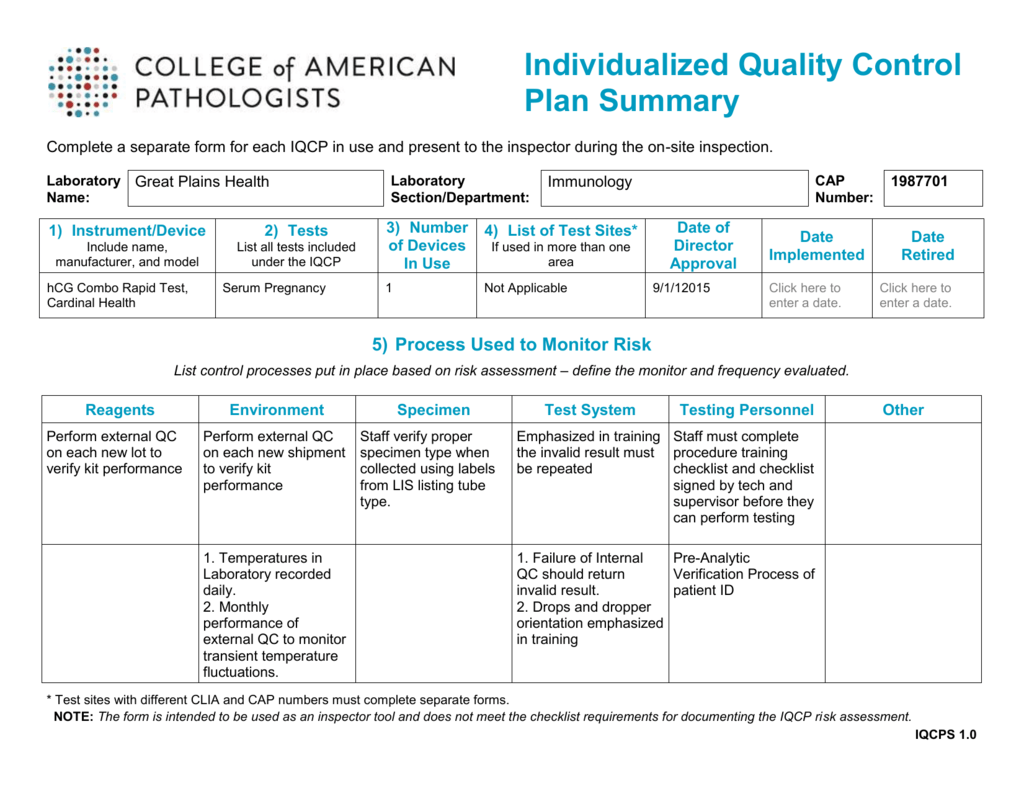
Individualized Quality Control Plan Summary
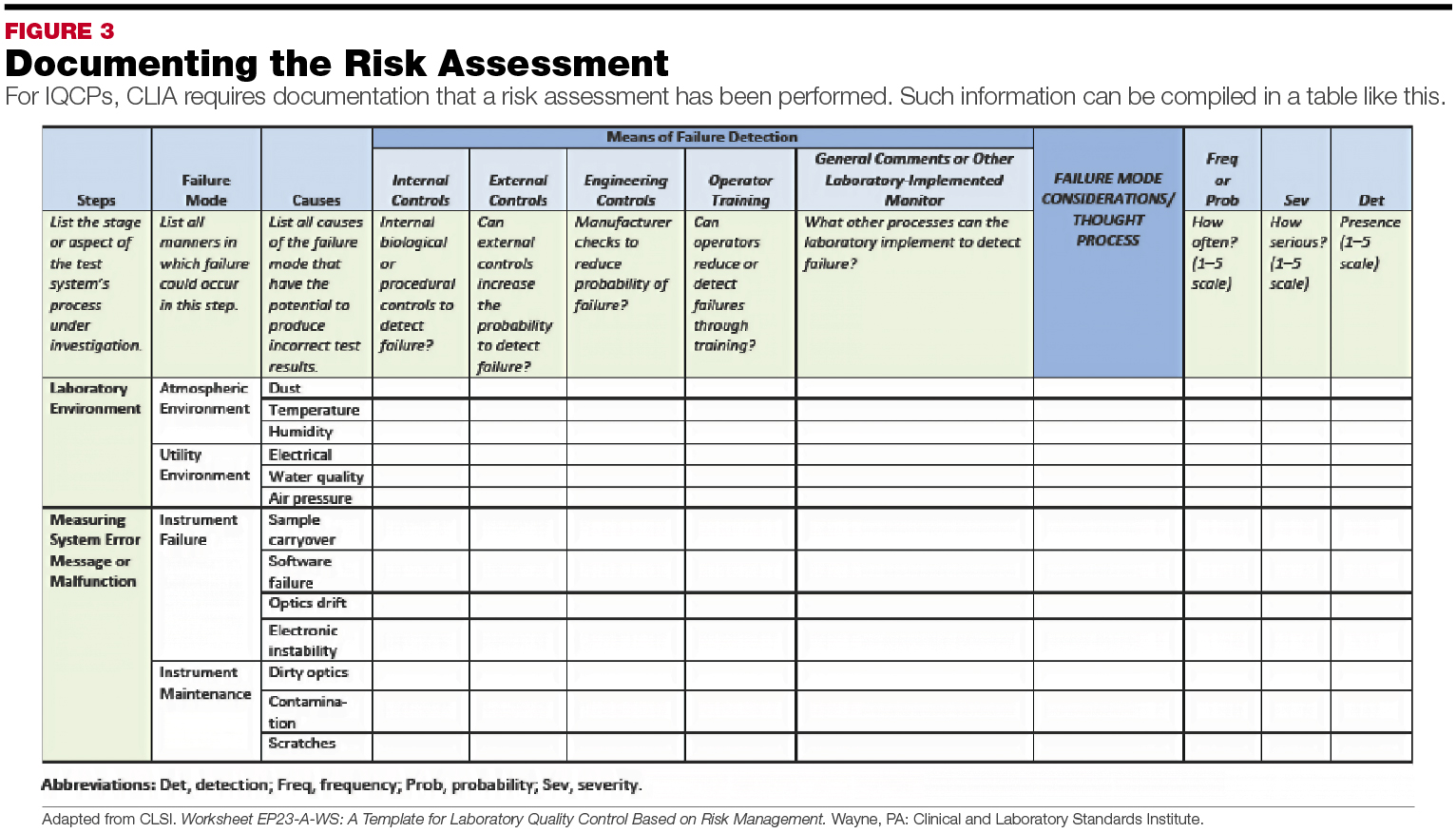
Develop Compliant IQCPs MarchApril 2015 MedicalLab Management Magazine
Example IQCP for C. Difficile Westgard QC
IQCP Guidelines and Template for Getting Started Linda C
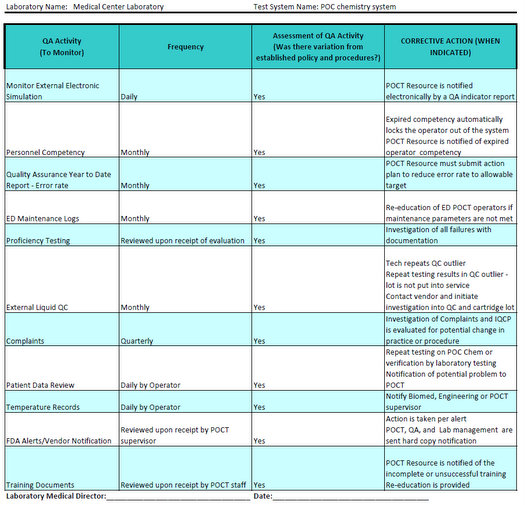
Example IQCP for POC chemistry system Westgard
Individualized Quality Control Plan (IQCP)
A Unique Iqcp Should Be Implemented For Each Separate Identification Method Or System.
A Distinguished Group Of Scientific Organizations Has Revised The Microbiology Individualized Quality Control Plan (Iqcp) Templates And Created The First Such Template For.
Learn How To Develop An Iqcp.
Learn About The Individualized Quality Control Plan (Iqcp) Option For Laboratories, Its Eligibility, Elements, And Benefits.
Related Post: