Note To File Template
Note To File Template - It ensures compliance with documentation standards. This template is provided by the office for the protection of research subjects (oprs) at the university of illinois. A note to file is a way to document deviations, problems, or events during human subjects research that cannot be recorded in other forms. [insert date of the ; If the issue relates to pi responsibilities (e.g., human subject. This web page provides a description of. Find templates and guidance for developing and conducting clinical research protocols, informed consent materials, and regulatory documents. Be signed and dated by the. Download a template for a standard note to file format and see examples of deviations, problems, and events to document. See a sample template and tips for clarity, conciseness, and signatures. This template is provided by the office for the protection of research subjects (oprs) at the university of illinois. This document provides guidance on how to create a note to file for human subjects research. If the issue relates to pi responsibilities (e.g., human subject. [insert date of the ; The study management templates are a university of michigan resource available to all study team members. Be signed and dated by the. Learn what a note to file is, when to use it, and how to write one. The templates are optional and can be customized to suit study team needs. If the issue relates to site performance, the appropriate credentialed individual from the site should write and sign the note to file. It ensures compliance with documentation standards. A note to file (ntf) may be used to: If the issue relates to site performance, the appropriate credentialed individual from the site should write and sign the note to file. The templates are optional and can be customized to suit study team needs. A template and examples are. It ensures compliance with documentation standards. It outlines when and how this documentation should be used. This template is provided by the office for the protection of research subjects (oprs) at the university of illinois. It ensures compliance with documentation standards. The study management templates are a university of michigan resource available to all study team members. If the issue relates to site performance, the appropriate. Download a template to document events or issues in a research study. Principal investigator/ investigator of record: [insert date of the ; If the issue relates to site performance, the appropriate credentialed individual from the site should write and sign the note to file. Include the subject and protocol to which it refers. Download a template for a standard note to file format and see examples of deviations, problems, and events to document. This web page provides a description of. It ensures compliance with documentation standards. Principal investigator/ investigator of record: The study management templates are a university of michigan resource available to all study team members. It outlines when and how this documentation should be used. Ote to file (ntf)] clinical research site. It ensures compliance with documentation standards. The purpose for this sop is to define the reason for crafting a note to file (ntf) and provide a note to study file template A note to file is a way to document deviations, problems, or. This web page provides a description of. Find templates and guidance for developing and conducting clinical research protocols, informed consent materials, and regulatory documents. Principal investigator/ investigator of record: This document provides guidance on how to create a note to file for human subjects research. A template and examples are. This template is provided by the office for the protection of research subjects (oprs) at the university of illinois. 2.1 all notes to the study file should be signed by the author, kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s documents and. Download a template for a standard note. It outlines when and how this documentation should be used. A note to file is a way to document deviations, problems, or events during human subjects research that cannot be recorded in other forms. Protocol number, version and date:. The purpose for this sop is to define the reason for crafting a note to file (ntf) and provide a note. The templates are optional and can be customized to suit study team needs. Download a template to document events or issues in a research study. See a sample template and tips for clarity, conciseness, and signatures. Principal investigator/ investigator of record: This file provides a template for creating a note to file, including specific guidelines and a sample note. • explain a discrepancy, the action taken in. The study management templates are a university of michigan resource available to all study team members. A note to file (ntf) may be used to: This template is provided by the office for the protection of research subjects (oprs) at the university of illinois. Protocol number, version and date:. This document provides guidance on how to create a note to file for human subjects research. Issuu turns pdfs and other files into interactive flipbooks and engaging content for every channel. The study management templates are a university of michigan resource available to all study team members. It ensures compliance with documentation standards. 2.1 all notes to the study file should be signed by the author, kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s documents and. The templates are optional and can be customized to suit study team needs. If the issue relates to site performance, the appropriate credentialed individual from the site should write and sign the note to file. The purpose for this sop is to define the reason for crafting a note to file (ntf) and provide a note to study file template • explain the location of a study document when it is not filed in the expected location; Include the subject and protocol to which it refers. It outlines when and how this documentation should be used. Learn what a note to file is, when to use it, and how to write one. See a sample template and tips for clarity, conciseness, and signatures. This file provides a template for creating a note to file, including specific guidelines and a sample note. Protocol number, version and date:. [insert date of the ;EXCEL of Simple Notes Description.xlsx WPS Free Templates
NoteToFile Template
Note To File Template Clinical Research
Meeting Notes Template 30+ Word, PDF Documents Download
Microsoft Word Note Taking Template For Your Needs
Legal File Note Template Notes template, Feedback for students, Book
Note To File Template Download by Pharma Student Issuu
Note Taking Template Word in 2022 Cornell notes template, Notes
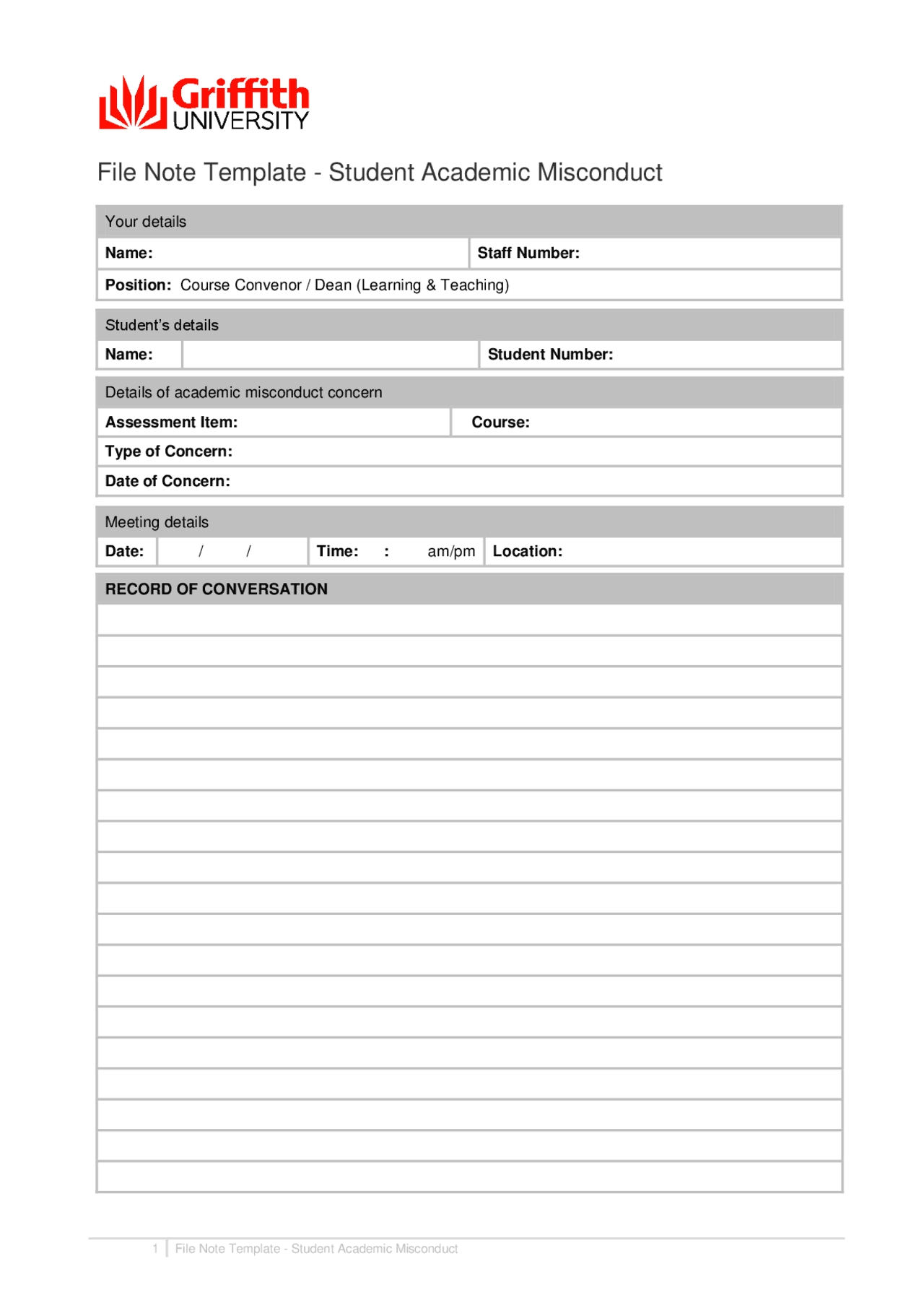
File Note Template Student Academic Misconduct Study Guides
Legal File Note Law Client Note Template Meeting Notes Telephone Note
This Template Is Provided By The Office For The Protection Of Research Subjects (Oprs) At The University Of Illinois.
Download A Template To Document Events Or Issues In A Research Study.
If The Issue Relates To Pi Responsibilities (E.g., Human Subject.
Principal Investigator/ Investigator Of Record:
Related Post: