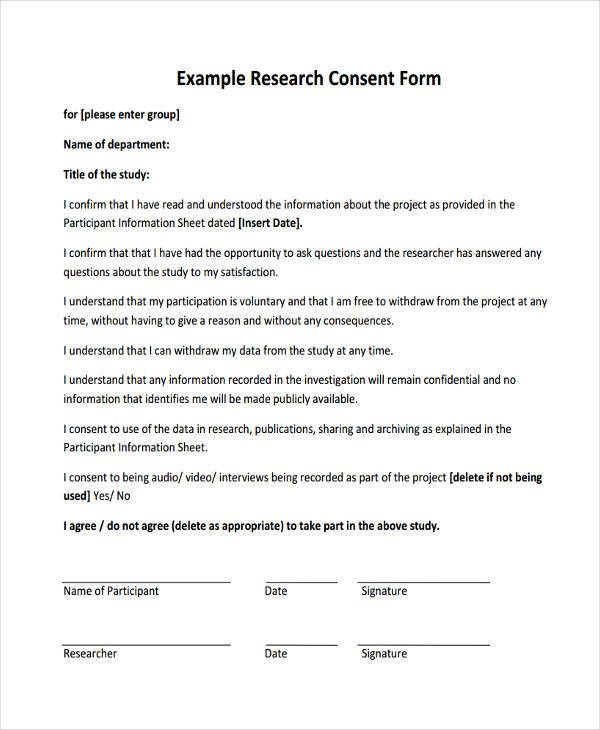
Research Consent Form Template
Research Consent Form Template - This template should be used as the consent document guide for all new research studies, including parental. Learn about the changes to the regulations for informed consent and the irb submission. Download the forms in doc format. Template participant consent form and participant information sheet providing information about the research to participants, and gaining consent from participants before their involvement is. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,. This consent should be used to obtain permission from subjects or the parent(s) of subjects (if minors will be. The consent form (icf) templates provided by the irb comply with federal regulations and hipaa. This resource provides points to consider and sample language for informed consent documents of research studies which plan to store and share data and/ or biospecimens for. There are other webpages devoted to providing guidance for writing readable,. Find templates and guidelines for consent and assent forms for various types of research, including biomedical, social, behavioral, and educational. The consent/assent form should be in a language that is understandable to someone without a scientific background. The consent form (icf) templates provided by the irb comply with federal regulations and hipaa. The templates are updated regularly and cover. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,. Investigators are required to use the latest versions of the informed consent form templates, which have been updated to comply with the 2019. Find templates for informed consent, assent, and debriefing forms for various types of human participant research studies. Learn how to use the new plain. Please use the microsoft readability statistics tool as needed when. Download the forms in doc format. This resource provides points to consider and sample language for informed consent documents of research studies which plan to store and share data and/ or biospecimens for. Find templates and guidelines for consent and assent forms for various types of research, including biomedical, social, behavioral, and educational. It is important that principal. Find consent form templates and guidance for different types of research projects. The consent form (icf) templates provided by the irb comply with federal regulations and hipaa. Includes tips, instructions, and examples for each element. This consent should be used to obtain permission from subjects or the parent(s) of subjects (if minors will be. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,. The templates are updated regularly and cover. The templates on this page are intended to. This template should be used as the consent document guide for all new research studies, including parental. The templates on this page are intended to help investigators construct documents that are as short as possible and written in plain language. Use these templates to properly inform prospective participants on scope of research informed consent for exempt research Consent document templates. There are other webpages devoted to providing guidance for writing readable,. Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for studies involving treatment, care, or diagnosis): The templates are updated regularly and cover. Informed consent and hipaa authorization form template v01nov2024 docx: The consent form (icf) templates provided by the irb comply with. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,. There are other webpages devoted to providing guidance for writing readable,. Oprs provides consent document templates as a resource tool for researchers. The templates are updated regularly and cover. Learn about the changes to. The consent/assent form should be in a language that is understandable to someone without a scientific background. Find templates for informed consent, assent, and debriefing forms for various types of human participant research studies. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,.. There are other webpages devoted to providing guidance for writing readable,. It is important that principal. Use these templates to properly inform prospective participants on scope of research informed consent for exempt research The templates on this page are intended to help investigators construct documents that are as short as possible and written in plain language. Include for studies that. It is important that principal. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed consent forms (icf). The templates are updated regularly and cover. Includes tips, instructions, and examples for each element of. The templates on this page are intended to help investigators construct documents that are. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,. Includes tips, instructions, and examples for each element of. Template participant consent form and participant information sheet providing information about the research to participants, and gaining consent from participants before their involvement is. The. Oprs provides consent document templates as a resource tool for researchers. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed consent forms (icf). Learn how to use the new plain. Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for studies. Download the forms in doc format. Learn about the changes to the regulations for informed consent and the irb submission. The consent/assent form should be in a language that is understandable to someone without a scientific background. Includes tips, instructions, and examples for each element of. Find templates and guidelines for consent and assent forms for various types of research, including biomedical, social, behavioral, and educational. This consent should be used to obtain permission from subjects or the parent(s) of subjects (if minors will be. There are other webpages devoted to providing guidance for writing readable,. Investigators are required to use the latest versions of the informed consent form templates, which have been updated to comply with the 2019. Oprs provides consent document templates as a resource tool for researchers. It is important that principal. Learn how to use the new plain. Informed consent and hipaa authorization form template v01nov2024 docx: This template should be used as the consent document guide for all new research studies, including parental. Use these templates to properly inform prospective participants on scope of research informed consent for exempt research Find templates for informed consent, assent, and debriefing forms for various types of human participant research studies. Consent document templates can be found on forms, guidance, and resources, by clicking the templates tab.FREE 12+ Research Consent Form Samples, PDF, MS Word, Google Docs
Research Consent Form Fill Out, Sign Online and Download PDF
FREE 12+ Research Consent Form Samples & Templates
FREE 12+ Research Consent Form Samples & Templates
Free Research Informed Consent Form PDF Word eForms
FREE 12+ Research Consent Form Samples & Templates
FREE 6+ Research Consent Forms in PDF MS Word
FREE 12+ Research Consent Form Samples, PDF, MS Word, Google Docs
FREE 12+ Research Consent Form Samples & Templates
FREE 6+ Research Consent Forms in PDF MS Word
Please Use The Microsoft Readability Statistics Tool As Needed When.
The Templates Are Updated Regularly And Cover.
Please Note That These Are Templates Developed By The Who Erc To Assist The Principal Investigator In The Design Of Their Informed Consent Forms (Icf).
This Resource Provides Points To Consider And Sample Language For Informed Consent Documents Of Research Studies Which Plan To Store And Share Data And/ Or Biospecimens For.
Related Post: